Metal Oxide Acid Salt Water Examples
Get the results you want.

Metal oxide acid salt water examples. Since metals that are reactive form oxides that are stable some metal oxides must be electrolyzed to be reduced. This includes sodium oxide potassium oxide calcium. In chemistry a salt is an ionic compound that can be formed by the neutralization reaction of an acid and a base. Salts are composed of related numbers of cations.
Acid metal oxide. A reaction between an acid and a metal oxide to form a salt and water as the only products. This is a special example of an acid base reaction. D glucose units swells in hot water creating a gel network.
Carbopol a polyacrylic acid swells when neutralized see figure 6. Bentone clays swell when. Acid definition a compound usually having a sour taste and capable of neutralizing alkalis and reddening blue litmus paper containing hydrogen that can be replaced. Oxyacid any oxygen containing acid.
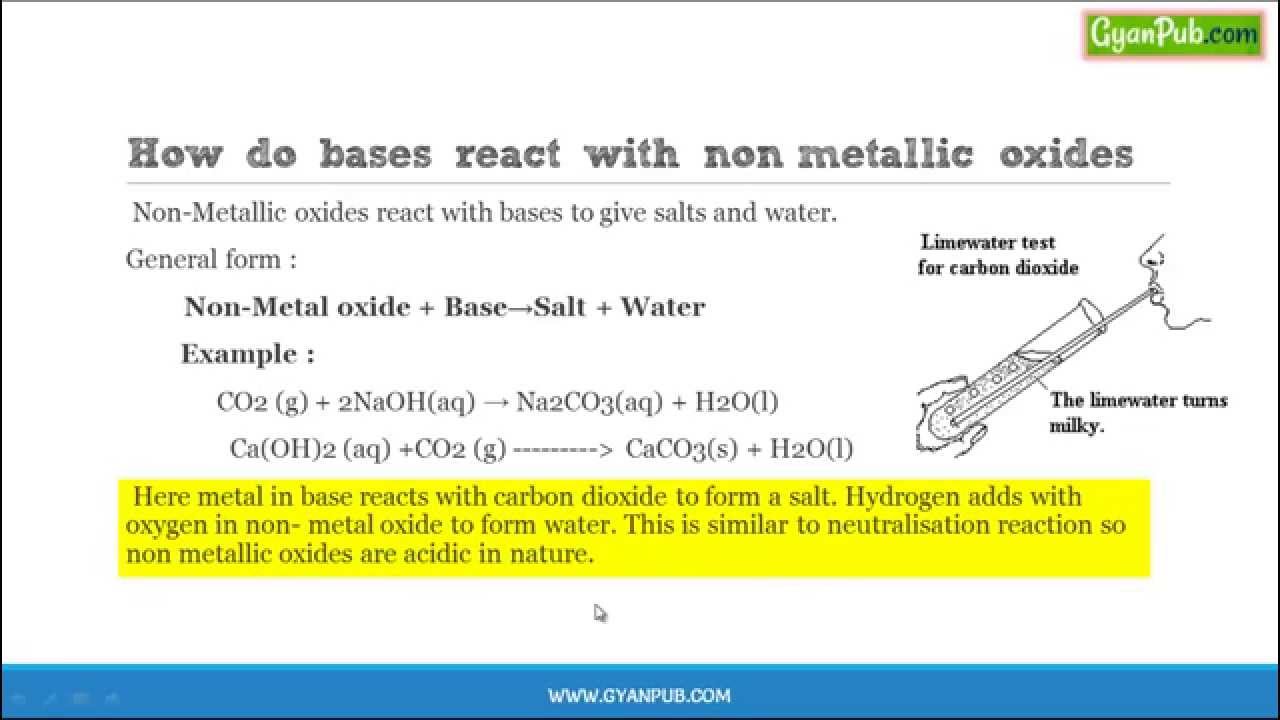
Most covalent nonmetallic oxides react with water to form acidic oxides. That is they react with water to form oxyacids. Packaging 1 l in glass bottle 1 1107 4 kg in glass bottle 1006 10075 101 ml in ampule 10 25 100 250 500 g in glass bottle. Facts answers information on water softeners and links to water softener comparisons and reviews.